Introduction
In July 2025, the FDA approved PTC Therapeutics' Sephience® (sepiapterin), providing a new oral cofactor replacement therapy for Phenylketonuria (PKU). This article analyzes the genetic basis of PKU, the mechanism of action for Sephience, and highlights advancements from the ASGCT 2025 meeting, focusing on AAV gene therapy strategies (like GS1168) that use a "dual-effect" approach of allele-specific silencing and functional reconstruction, aiming for a "one-time cure."
Genetic Mechanism of PKU: PAH Gene Mutations and Phenylketonuria Treatment Targets
Phenylketonuria (PKU) is an autosomal recessive rare disease, caused by mutations in the PAH (Phenylalanine Hydroxylase) gene.
The PAH gene (located at 12q22-q24.2) encodes the liver-specific enzyme phenylalanine hydroxylase (PAH), which converts the essential amino acid phenylalanine (Phe) into tyrosine. This process is highly dependent on its cofactor, tetrahydrobiopterin (BH4).
Over 1,000 pathogenic PAH variants have been reported. These diverse mutations (missense, nonsense, splicing, deletions) lead to a partial or complete loss of PAH enzyme function. When PAH activity is insufficient, Phe accumulates in the body, causing neurotoxicity and irreversible intellectual disability.
Notably, a significant portion of PKU patients carry missense mutations that reduce the PAH protein's affinity for BH4 or decrease its stability. This mechanism is the molecular basis for "BH4-responsive PKU" and the theoretical foundation for cofactor replacement therapies.
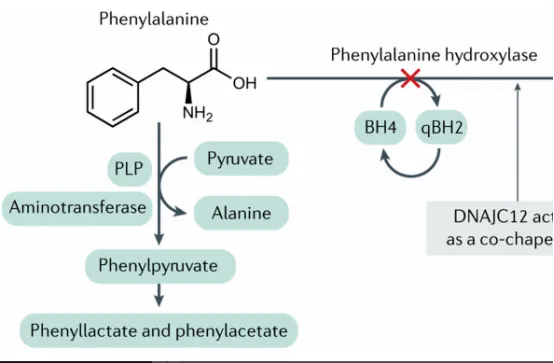
New Drug for Phenylketonuria Treatment: The Cofactor Mechanism of Sephience (Sepiapterin)
The FDA's approval makes Sephience a crucial addition to phenylketonuria treatment. It is an oral natural BH4 precursor replacement.
Mechanism of Action
Sepiapterin is absorbed and converted to BH4 via the endogenous salvage pathway, bypassing the rate-limiting steps in BH4 synthesis. This more effectively increases intracellular BH4 levels in hepatocytes, which is particularly critical for patients whose PAH mutations reduce enzyme-cofactor affinity. By providing sufficient cofactor, Sephience "activates" and stabilizes residual PAH activity, restoring the Phe metabolic pathway.
Clinical Evidence
Data from the pivotal Phase 3 APHENITY clinical trial (NCT04849767) showed that Sephience treatment significantly reduced plasma Phe levels in PKU patients, with an average reduction exceeding 30%.
The Frontier of PKU Gene Therapy: The "One-Time Cure" Dual-Effect Strategy (GS1168)
Although cofactor replacement therapy has advanced, it remains a palliative treatment. Therefore, achieving a "one-time cure" with PKU gene therapy has become the core research frontier.
At the 2025 American Society of Gene & Cell Therapy (ASGCT) meeting, research on gene therapy for PKU showed exciting breakthroughs. For example, the candidate drug GS1168, reported by Huayi-LeHealth, gained attention for its innovative "dual-effect" strategy.
The Core Challenge
A key challenge is that in many patients, the mutant PAH protein (mutant PAH) exerts a Dominant Negative Effect on the wild-type PAH expressed by the AAV vector.
GS1168's Dual-Effect Mechanism
To solve this, GS1168 integrates two mechanisms into a single AAV vector:
- Break Interference (Allele-Specific Silencing): The vector expresses an optimized microRNA (miRNA) module designed to specifically recognize and silence the patient's endogenous, mutant PAH mRNA.
- Rebuild Function (RNAi-Resistant Expression): The vector simultaneously expresses a codon-optimized and RNAi-resistant PAH protein. Its sequence is synonymously mutated to evade the miRNA, achieving the dual goal of silencing the mutant gene while expressing the therapeutic one.
Research Implications: Future Directions and Animal Models for PKU Gene Therapy
From Sephience's approval to GS1168's innovation, the treatment paradigm for PKU is shifting from "metabolic control" to "genetic repair." This provides key insights for researchers:
- Allele-Specific Manipulation is Key: The strategy of allele-specific silencing combined with functional replacement (as in GS1168) offers a translatable R&D concept for other genetic diseases facing similar dominant-negative challenges (e.g., in liver or neurological disorders).
- Precise Animal Models are Crucial: To validate complex dual-effect AAV vectors like GS1168, researchers must use PKU animal models that mimic the human dominant-negative effect (e.g., Pah knock-in mice with specific point mutations), rather than simple Pah knock-out (KO) models.
Content Source and Disclaimer
This article is a compilation and interpretation of recent industry developments (including FDA approvals and the ASGCT 2025 meeting), intended to highlight scientific progress in the field. All information is based on public data and the cited references.






