Toolbox
Mutation Analysis Tools
AI Tool
RNA Splicer
AI tool for predicting the impact of gene mutations at any location on RNA splicing sites
AI Tool

Pathogenicity Predictor
AI tool for predicting the pathogenicity of gene mutations at any location
Mutation Direct
Perform joint or cross searches for mutations, genes, and diseases
SNP Viewer
Access mutation information of target genes from the dbSNP database in a visual format
AI Tool
Mouse Pheno Predictor
AI tool for predicting the impact of specific mutations on mouse phenotypes
ASO Designer
Design antisense oligonucleotides by calculating binding affinity to target RNA.
Sequence Analysis Tools
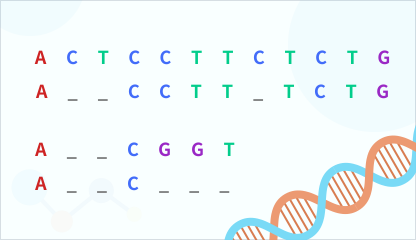
Sequence Alignment
Compare nucleotide or amino acid sequence similarities
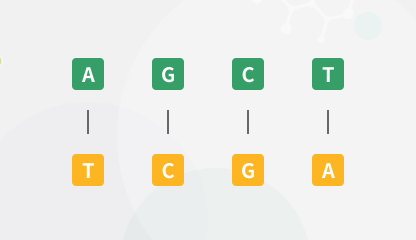
Reverse Complement Converter
Convert the input sequence to its reverse, complement, or reverse complement sequence
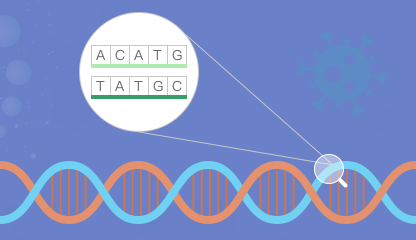
Sequence Viewer
Access detailed information on cDNA, RNA transcripts, and corresponding protein sequences for specific genes
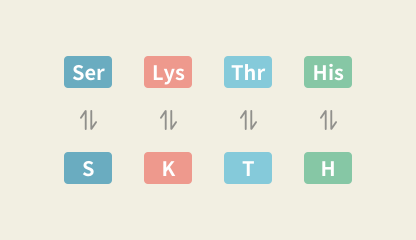
Amino Acid Abbreviation Converter
Convert amino acids to and from their symbols
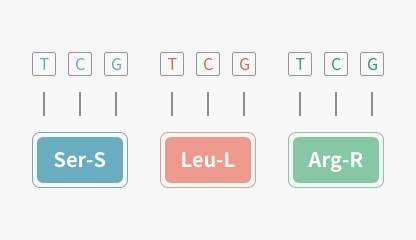
Sequence Translator
Translate DNA or RNA sequences into amino acid sequences
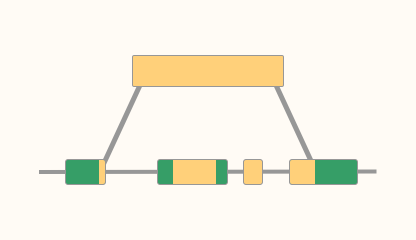
BLAT
A BLAST-like tool: find nucleotide sequences with over 95% similarity
Comparison Tools
Other Tools
AI Tool
AI Assistant
Professional AI assistant in the field of biology
AI Tool
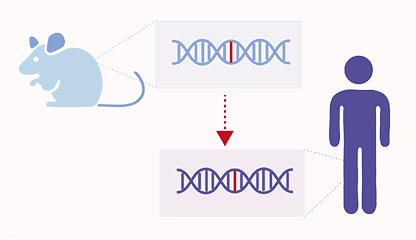
Homolog Predictor
AI tool for predicting human homolog gene changes based on mouse gene expression data
Pathway Enrichment Analysis
Pathway enrichment analysis on gene expression data
Ensembl-NCBI Converter
Convert gene IDs between Ensembl and NCBI
AI Tool
Gene Expression Database
AI-powered database for visualizing specific gene expression based on singlecell sequencing literature
Cancer Target Database
Query the response of specific cancer targets to drugs
AI Tool
Affinity Predictor
AI tool for predicting protein complex binding affinity