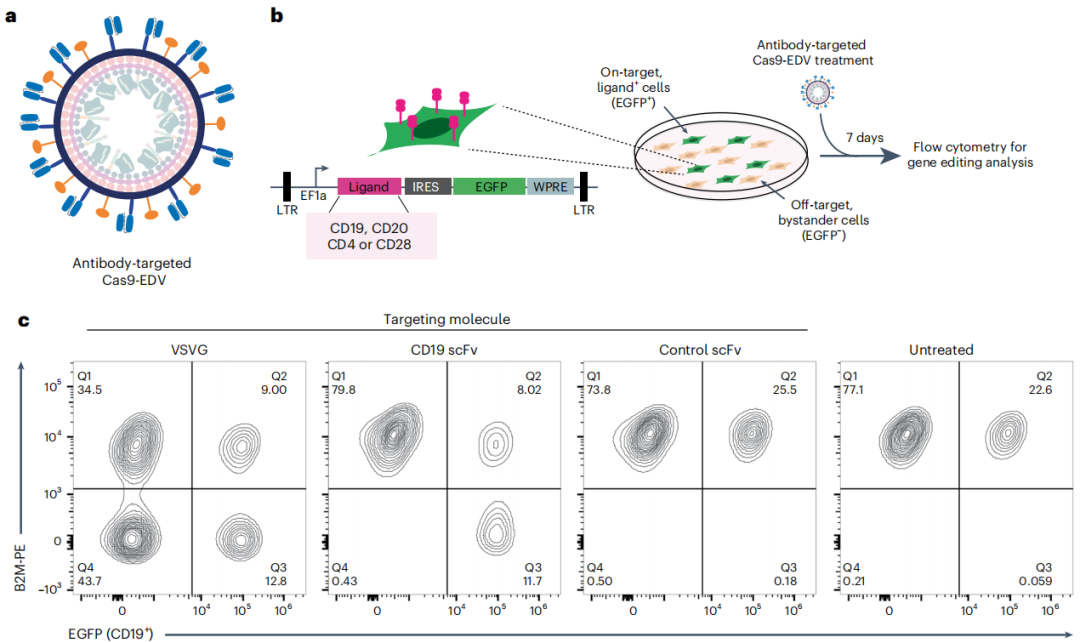
Figure 2: Cell-specific genome editing with antibody-targeted Cas9-EDVs. Source: Nature Biotechnology
Nature Biotechnology: Another masterpiece from a Nobel laureate! Doudna's team develops a new method to deliver genome editing tools to specific cells.
Viruses and virus-derived particles possess an intrinsic ability to deliver molecules to cells, but the difficulty in altering their selectivity for cell types has hindered their use for therapeutic delivery.
On January 14, 2024, the team led by Jennifer A. Doudna at the University of California, Berkeley, published a research paper titled "In vivo human T cell engineering with enveloped delivery vehicles" in Nature Biotechnology online. This study demonstrated that by displaying antibody fragments on the surface of membrane-derived particles encapsulating CRISPR-Cas9 proteins and guide RNA, which recognize cell surface markers, genome editing tools can be delivered to specific cells.
Compared to traditional vectors such as adeno-associated viruses that rely on evolutionarily predisposed capsid tropisms to deliver virus-encoded cargoes, these Cas9-packaged enveloped delivery vehicles (Cas9-EDVs) utilize predictable antibody-antigen interactions to selectively and transiently deliver genome editing mechanisms to cells of interest. Antibody-targeted Cas9-EDVs preferentially confer genome editing capabilities to homologous target cells within mixed populations in vitro and in vivo, rather than to bystander cells. By directly delivering to human T cells using multiple targeting molecules, Cas9-EDVs are capable of generating genome-edited chimeric antigen receptor T cells in humanized mice, establishing a programmable delivery method with broad therapeutic potential.
Figure 1 Source: Nature Biotechnology
Therapeutic interventions involving genome editing require the safe and effective delivery of molecules into the nuclei of target cells. Although this capability would transform clinical and research applications, current non-viral delivery is limited to cells treated ex vivo, tissues subjected to local administration, or the liver, due to their natural propensity for molecular uptake. Recent lipid nanoparticle formulations have been described as having affinity for non-hepatic cells or organs, but expanding in vivo genome editing applications may require multiple approaches to deliver molecules to specific cells or organs within the body after systemic administration.
Redirecting the tropism of viruses or viral vectors is a well-established delivery strategy that involves the surface display of cell-selective targeting molecules, as well as viral glycoproteins required for entry into cells through fusion in the low pH environment of the plasma membrane or endosomes. Recent advances have utilized a mutated form of the Vesicular Stomatitis Virus Glycoprotein (VSVG), VSVGmut, which retains endosomal fusion activity but lacks the natural affinity for binding to low-density lipoprotein receptors. Pairing VSVGmut with cell-specific targeting molecules can redirect the transgene delivery of lentiviruses and enable high-throughput screening of T-cell and B-cell receptor libraries to study receptor-antigen interactions.
Particles hidden within cell membrane fragments, such as retrovirus-like particles (VLPs), extracellular vesicles, and biomimetic nanoparticles, are becoming increasingly popular for the delivery of molecular cargo. For these types of engineered delivery vehicles (EDVs), bioengineering is required to achieve packaging of molecular cargo as well as control of targeting and fusion activities. It has been demonstrated that by pairing VSVGmut with antibody-derived single-chain variable fragments (scFvs) on the EDV carrying the Cas9 ribonucleoprotein (RNP) complex (Cas9-EDV), human cell-specific genome editing can be achieved both in vivo and in vitro.
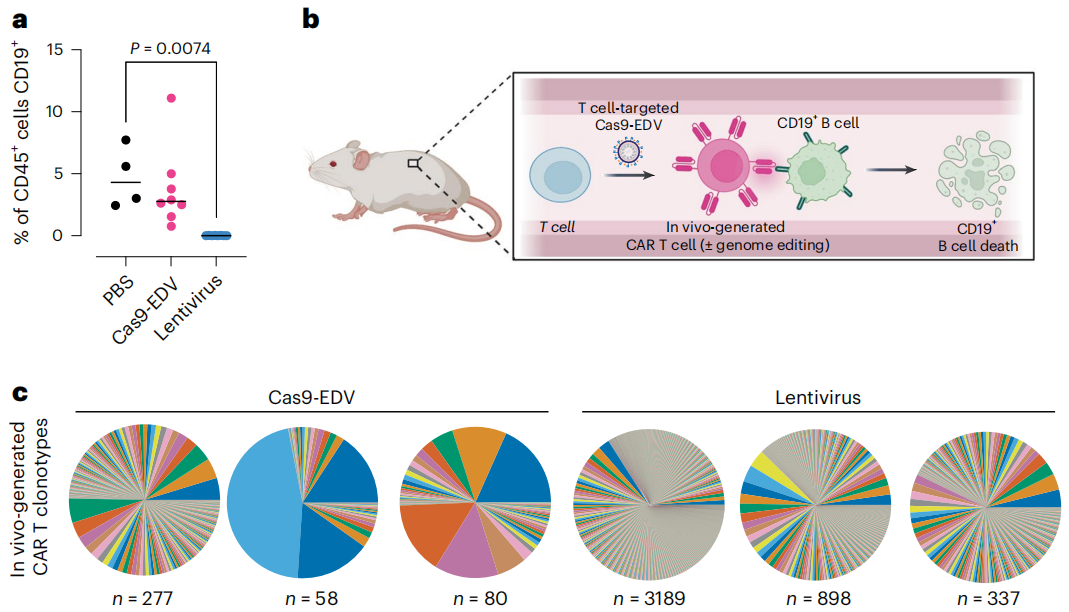
Figure 3: Functional dynamics of in vivo cell engineering. Source: Nature Biotechnology
The study described engineered delivery vehicles (EDVs) that utilize retrovirus-like particle (VLP) assembly for the transient delivery of Cas9 ribonucleoproteins (RNPs). The research found that Cas9-EDVs achieved targeted genome editing in chimeric antigen receptor (CAR) T cells produced in mice with humanized immune systems, without off-target delivery to hepatocytes. These data suggest that EDVs represent a programmable platform capable of delivering molecular cargo to specific cell types for complex in vivo genome engineering, including gene delivery and targeted gene disruption. This approach opens up new possibilities for precision medicine, allowing for the targeted modification of genes in specific cell populations, potentially leading to more effective and safer therapeutic interventions.
Link to the paper:https://www.nature.com/articles/s41587-023-02085-z
Source: Biological Exploration
[Disclaimer] This article is a repost. This platform is only for sharing and disseminating information. All copyrights belong to the original author. If there is any infringement, please contact us to remove it.